InjecTIon molding an IC into a connector or consumable item
InjecTIon molding is the method of choice to embed integrated circuits (ICs) in medical sensors and consumables. This applicaTIon note discusses the special care required when selecTIng the plastic material. It explains that the processing temperature must be low enough not to soften or liquefy the solder that attaches the IC to a substrate. The article also discusses various plastic materials and shows how they are not all equally suited for the sterilization required by medical applications. Only few of the sterilization methods are compatible to both the plastic material and ICs. An example of an IC embedded into a consumable item is shown.
Integrated circuits (ICs) have been embedded in medical sensors and consumables or cables for quite some time. The concept and benefits of this technique are well understood.1 However, very few companies have acquired the expertise for both the manufacturing process and selecting suitable plastics for the application. This document describes the injection molding process in general, explains the critical temperatures, and gives an introduction to identifying suitable plastic materials.
Injection molding is a process that forces molten plastic through a nozzle into a mold cavity. The mold cavity can initially be empty or contain an object to be encased by the plastic. Figure 1 shows a simplified drawing of an injection molding machine.2 The plastic is fed into the machine as granules. A screw-type plunger transports the granules through a heated barrel towards the nozzle. Along the barrel there are typically three heating zones called rear, center, and front. The front zone, next to the nozzle, is the hottest. On the way to the nozzle the plastic granules soften and become a homogenous mass, which is forced under high pressure into the mold cavity, where it quickly cools and hardens. The mold is then opened to remove the molded object and to prepare the mold for the next cycle. The processing conditions specification in the data sheet for the plastic material lists the appropriate temperatures of the zones and the mold.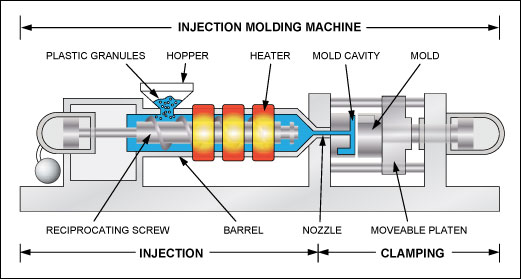
Figure 1. The principle of an injection molding machine.
Besides pressure and filling speed, temperature is a critical parameter in the injection molding process. Temperature is also critical for the object to be encased. An IC by itself can handle up to 300°C for a short time without any harm. This temperature is permissible if the IC to be encased is wired-bonded to a substrate (circuit board) or if it is a plastic-packaged IC with crimped-on pins or in an SFN package3 with exposed contact areas. However, if the IC is soldered to a circuit board, the critical temperature for injection molding depends on whether the IC assembly is lead (Pb)-free or not. Lead-free ICs (plastic packaged or bumped die, also known as flip chip, UCSP™, or WLP) require lead-free solder, which has a melting temperature of approximately 217°C. Traditional plastic-packaged ICs and RoHS-compliant bumped dies require standard solder (Sn/Pb 63/37), which has a melting temperature of approximately 183°C. The melting temperature must not be confused with the reflow oven temperature setting, which is 235°C for lead-free and 215°C for standard products.
With roughly 30,000 different materials available for injection molding,4 finding the right plastic may appear like a serious challenge. The task becomes easier with some insight into the characteristics of various plastic materials.5
There are thermosetting plastics, which can be heated and run through an injection molding machine just once. Epoxy, a plastic commonly used for IC packages, falls into this category. Once processed, the thermosetting plastic remains solid, regardless of the temperature. There are thermoplastics, which soften whenever they are heated. Thermoplastics can have an amorphous or semi-crystalline structure, which affects mechanical stability, chemical/wear resistance6, 7 and suitability to sterilization methods. Table 1 shows a selection of plastic materials for injection molding.
Table 1. Typical plastics for injection molding
49 to 85 ETO CD RAD
171 to 199 21 to 66
21 to 52 ETO CD (rad)
249 to 271 66 to 107 AUT ETO CD VHP
216 to 232 66 to 121 AUT ETO RAD
216 to 232 82 to 121
66 to 121 AUT ETO (rad)
221 to 238 79 to 107
38 to 66 ETO CD VHP
177 to 199 82 to 121 AUT ETO
177 to 193 32 to 66 AUT ETO CD VHP
204 to 221 38 to 66 ETO CD VHP RAD
210 to 232 52 to 82
38 to 79 —
Legend:
AUT: material is well suited for autoclave sterilization.
ET material is well suited for etylene oxide sterilization.
CD: material is well suited for chlorine dioxide sterilization.
VHP: material is well suited for vaporized hydrogen peroxide sterilization.
RAD: material is well suited for gamma ray or electron beam sterilization.
(rad): material may change its characteristics, e.g., discolor, when exposed to gamma rays or electron beams.
The temperature values above are compiled from data on the RTP Company11 website. Each plastic type usually has several variations with different processing and mold temperatures. Other vendors may offer the same plastic type with slightly different processing requirements. Additives such as reinforcement fibers, pigment, flammability reduction compounds, or conductivity enhancers can also impact the processing conditions. As a guideline, one should select the plastic based on the nozzle temperature or the front zone temperature as specified in the processing conditions for injection molding of plastics. For injection molding at 183°C, the choice of plastic is very limited. At 217°C, most of the materials in Table 1 are feasible. Materials such as nylon (PA) or polyethylene terephthalate (PET) can be used only if the IC is not soldered to a substrate. The plastic cools off rapidly inside the mold cavity. In summary, one should make sure that when the plastic enters the mold, it does not exceed the critical temperature.
Sterilization compatibility
Products for medical applications typically undergo sterilization, at least once or even multiple times during their lifetime. Popular methods are autoclave steam sterilization, ethylene oxide (ETO) sterilization, chlorine dioxide (CD) sterilization, vaporized hydrogen peroxide (VHP) sterilization, hydrogen peroxide plasma sterilization, and irradiation with gamma rays or electron beams. The sterilization compatibility information in Table 1 is compiled from various publications. A report on hydrogen peroxide gas-plasma compatibility was not found. In any case, caution is advised since the test methods and details are not fully disclosed. For objects with an embedded IC the preferred sterilization methods are ETO and CD. Autoclave and VHP may also be acceptable, although the high temperature or vacuum required by these methods can affect embedded batteries or floating-gate memory cells. Hydrogen peroxide gas-plasma sterilization and irradiation (gamma ray or electron beam) are not recommended since they can damage ICs.
If the temperature of the plastic is too high when entering the mold, the solder that holds the IC in place softens or liquefies. As this hot plastic enters the mold cavity it can push the IC away from the pads on the substrate. Solder can protrude from the mold or short IC terminals, making the end product unusable or unreliable. Therefore, choosing the right temperature is a very critical step.
Nevertheless, there could be situations that require a plastic with a processing temperature above the solder melting point. In that case, one can consider two molding steps: first, use a plastic that stays below the critical temperature; second, use the plastic that requires the otherwise unacceptable temperature. Thus, with properly chosen dimensions and a well-timed temperature profile, the thermal inertia of the first layer protects the IC from the excess heat of the second molding step.
Application note 47021 features a DB9 connector with an embedded TO92-packaged 1-Wire® device. In this case, two of the DB9 pins are crimped to the IO and GND leads of the TO92 package. The NC (not connected) lead is cut off. This construction maximizes the choice of plastics since no solder is involved. Figure 2 shows the same connector before molding. The TO92 package is easily recognized.
Figure 2. DB9 connector with embedded TO92 1-Wire device before injection molding.
Injection molding is the method of choice to embed ICs in medical sensors and consumables. Special care is required when selecting the plastic material since its processing temperature must be low enough not to soften or liquefy the solder that attaches the IC to a substrate. Plastic materials are not equally suited for the sterilization required by medical applications. Consequently, only few of the sterilization methods are compatible with both the plastic material and ICs.
References
- See Maxim application note 4702, "Easily add memory, security, monitoring, and control to medical sensors and consumables," and application note 4623, "Smart cable aids quality control and authentication."
- Injection molding, http://en.wikipedia.org/wiki/Injection_molding
- For further reading, see Maxim application note 4132, "Attachment methods for the electro-mechanical SFN package."
- For examples of polymers best suited for the process, go t http://en.wikipedia.org/wiki/Injection_molding.
- For a discussion of the Characteristics of Various Plastic Materials, go t http://www.fcs.com.tw/webtop/FAQ_EN/index.phtml?action=browse&id=170&StoreID=9&, Fu Chun Shin Machinery Manufacture Co. Ltd.
- Choosing the Correct Thermoplastic Composite, RTP Company, at: http://www.thomasnet.com/white-papers/abstract/100711/choosing-the-correct-thermoplastic-composite.html.
- Selection of Materials for Medical Applications, RTP Company, at: http://www.thomasnet.com/white-papers/abstract/100710/selection-of-materials-for-medical-applications.html.
- Sterilization of Plastics, Zeus Industrial Products, at: http://www.thomasnet.com/white-papers/abstract/101488/sterilization-of-plastics.html.
- Gaseous Chlorine Dioxide and the Myth of Corrosion at: http://www.clordisys.com/MythOfCorrosion.pdf.
- Material Compatibility with Vaporized Hydrogen Peroxide (VHP®) Sterilization, http://www.steris.com/media/PDF/spacedecon/pharmaSolutions/point%20of%20manufacture%20mat%20comp%20pharma%20case%20study%202.pdf.
- Injection molding Processing Conditions for RTP Specialty Compounds, RTP Company, at: http://www.rtpcompany.com/info/processing/index.htm.
1-Wire is a registered trademark of Maxim Integrated Products, Inc.
UCSP is a trademark of Maxim Integrated Products, Inc.
欢迎分享,转载请注明来源:内存溢出

 微信扫一扫
微信扫一扫
 支付宝扫一扫
支付宝扫一扫
评论列表(0条)